
Meet the 2023 FDA Cybersecurity Requirements
From software bills of materials to integrated device security, DigiCert solutions can help you meet current FDA requirements—with the agility to quickly comply with any future updates.
Device Security by Design
In September of 2023, the FDA released new requirements for medical device cybersecurity practices and information to be provided during premarket submissions. The requirements are broken down into four general principles:

Cybersecurity is part of device safety and the quality system regulation quality system regulation
Submissions must demonstrate a “reasonable assurance of safety and effectiveness,” which includes software validation and risk management as defined by a strong Secure Product Development Framework (SPDF) that encompasses all phases of a device’s lifecycle.

Premarket submissions should clearly define how the following security objectives are addressed by and integrated into the device design:
- Authenticity
- Authorization
- Availability
- Confidentiality
- Secure and timely updatability and patchability

Transparency
Device users should have clear and accessible information about the device’s cybersecurity controls, potential risks, known vulnerabilities, configuration and update instructions, and other pertinent information spanning the device’s entire lifecycle.

Documentation
Cybersecurity design and documentation should scale with the risk level of the device, taking into account the larger system in which the device will be used. Generally, the more connected and integral the device, the stronger and more comprehensive the documentation is expected to be.
Optional heading that can be visually hidden
Achieve Compliance With End-to-End Trust and Device Lifecycle Management
5 key benefits of DigiCert device trust solutions for healthcare IoT
- Meet compliance guidance with end-to-end device lifecycle management
- Embed and control device security from chip to field
- Quickly produce comprehensive Software Bills of Materials (SBOMs)
- Identify threats, monitor vulnerabilities and prevent certificate outages
- Discover and manage your entire certificate ecosystem, regardless of the issuing authority
Seamless Integration
DigiCert solutions are designed to integrate into the stack to provide full lifecycle visibility, security, and control.
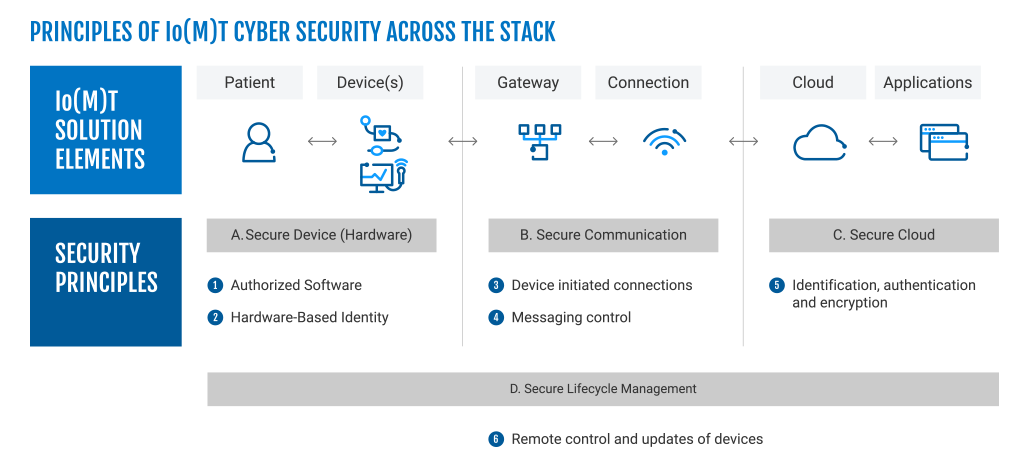

New FDA Requirements for IoMT
Read the newest pre-market requirements from the FDA.



